propene molecular geometry|bond energy of c3h8 : Tuguegarao C3H6 Lewis structure contains three carbon atoms bonding with six hydrogen atoms, all these are bonded with the 7 single . Tingnan ang higit pa IndyMill is an open-source CNC machine project developed by Nikodem Bartnik as an upgrade to the Dremel CNC. The goal is to create a DIY CNC machine that is easy to build and replicate worldwide. By using widely available .
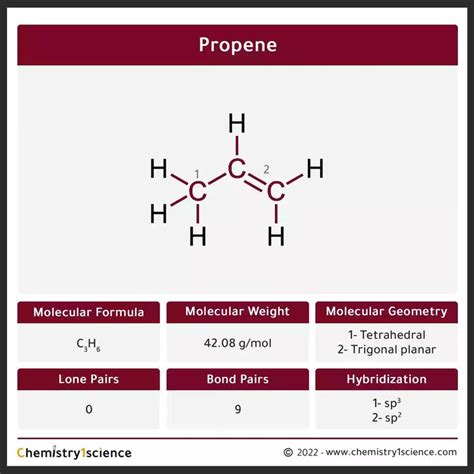
propene molecular geometry,C3H6 has two types of molecular geometry, trigonal planar and tetrahedral. Look at the lewis structure of C3H6, we have three carbon atoms. ⇒ The right side carbon is attached with four bonded pairs, which means, it forms AX4 type that implies its geometry will be tetrahedral. ⇒ The middle and left . Tingnan ang higit paC3H6 Lewis structure contains three carbon atoms bonding with six hydrogen atoms, all these are bonded with the 7 single . Tingnan ang higit pa
1. Count total valence electron in C3H6 Finding the total valence electrons in the molecule is our topmost priority for drawing . Tingnan ang higit paIs Propene (C3H6) polar or non-polar? Propene (C3H6) is a nonpolar molecule because the difference in electronegativity between carbon (2.55) and hydrogen (2.2) is less . Tingnan ang higit pa
In C3H6, the two carbon atoms (those that have a double bond between them) are Sp2 hybridized and one carbon that attached only four single bonds is Sp3 hybridized. So, the hybridization of Propene . Tingnan ang higit paprop-1-ene. 1-Propene. Methylethylene. View More. Molecular Weight. 42.08 g/mol. Computed by PubChem 2.2 (PubChem release 2021.10.14) Dates. Create: 2004-09-16. Modify: 2024-03-30. Description. Propylene .this record. 3D. Propene. Molecular Formula CH. Average mass 42.080 Da. Monoisotopic mass 42.046951 Da. ChemSpider ID 7954. More details: Featured data source. Names. Properties. Searches. Spectra. Vendors. . Name of Molecule . Propene . Molecular Geometry . 1- Tetrahedral. 2- Trigonal planar . Hybridization . 1- sp 3. 2- sp 2 . .
1-propene. Molecular Model. Jmol._Canvas2D (JSmol) "jmolApplet0" [x] appletId:jmolApplet0_object (signed) starting HoverWatcher_1. getValue emulate = null. .
Propene-d6; Other names: Propylene; 1-Propene; Methylethylene; 1-Propylene; CH3CH=CH2; Methylethene; NCI-C50077; UN 1077; R 1270 Permanent link for this .propene molecular geometry bond energy of c3h8Propene. Formula: C 3 H 6. Molecular weight: 42.0797. IUPAC Standard InChI: InChI=1S/C3H6/c1-3-2/h3H,1H2,2H3. Copy Sheet of paper on top of another sheet. . A quick explanation of the molecular geometry of C3H8 (Propane) including a description of the C3H8 bond angles. Looking at the C3H8 Lewis structure .Polymerization. Dimerization. Environmental safety. Storage and handling. Occurrence in nature. See also. References. Propylene, also known as propene, is an unsaturated .
The PROPYLENE molecule contains a total of 8 bond (s). There are 2 non-H bond (s), 1 multiple bond (s), and 1 double bond (s). Images of the chemical structure of PROPYLENE are given below: 2-dimensional (2D) .Figure 10.2.2 ): (CC BY-NC-SA; anonymous) The two oxygens are double bonded to the sulfur. The oxygens have 2 lone pairs while sulfur had one lone pair. 3. There are two bonding pairs and one lone pair, so the structure is designated as AX 2 E. This designation has a total of three electron pairs, two X and one E. A step-by-step explanation of how to draw the C3H6 Lewis Structure.For this chemical formula there are two different ways to draw the C3H6 Lewis structure. .
Using VSEPR theory, predict the molecular geometry of BrF5. a) Octahedral b) Square pyramidal c) T-shaped d) Trigonal bipyramidal e) None of the above; Describe the molecular geometry of BrF3. Using VSEPR THEORY explain why BF_4^- is a tetrahedral molecule. Using the valence bond theory (and VSEPR), predict the molecular .
For the following compound draw an appropriate Lewis structure, determine the molecular geometry using VSEPR theory, determine whether the molecule is polar and identify the hybridization of all interior atoms: IF_5; Draw and explain the Lewis structure of SeF2. Determine its molecular geometry, hybridization, bond angles, and polarity.
In this video we’ll write the structural formula for Propene (also called Propylene). Propene is a hydrocarbon consisting of Carbon (C) and Hydrogen (H) ato. Molecular Geometry: Molecules have a balanced geometric shape, the bonds have a certain length and angle as well, and the laws of quantum mechanics determine this. The chemical equation and the structural equation of a molecule are the two most important factors in determining its properties, especially its activity. .Propene-d6; Other names: Propylene; 1-Propene; Methylethylene; 1-Propylene; CH3CH=CH2; Methylethene; NCI-C50077; UN 1077; R 1270 Permanent link for this species. Use this link for bookmarking this species for future reference. Information on this page: Notes; Other data available: Gas phase thermochemistry data; Condensed phase .Molecular geometry is a way of describing the shapes of molecules. It applies a theory called VESPR for short. VESPR stands for valence shell electron pair repulsion. This theory basically says that bonding and non-bonding electron pairs of the central atom in a molecule will repel (push away from) each other in three dimensional space and this .
7 Molecular Geometry and Polarity Purpose. To study the molecular geometry and polarity of a range of molecules. Expected Learning Outcomes. . Consider the structure of propene: Lewis structure of propene. About carbon atoms 1 and 2, there are three electron groups about each of them, and all of them are bonding pairs.
{ "name":"jmolApplet0_object","applet":true,"documentBase":"https://www.chem.purdue.edu/jmol/molecules/c3h6.html","platform":"J.awtjs2d.Platform","fullName .
propene molecular geometryThe molecule itself may have a particular shape which is determined by the geometries of it constituent atoms, but which can't be described simply in words as "tetrahedral" or "linear". Pentane is roughly a zig-zag shape. In .Propene-d6; Other names: Propylene; 1-Propene; Methylethylene; 1-Propylene; CH3CH=CH2; Methylethene; NCI-C50077; UN 1077; R 1270 Permanent link for this species. Use this link for bookmarking this species for future reference. Information on this page: Gas phase thermochemistry data; Condensed phase thermochemistry data; .Molecular Weight. 44.10 g/mol. Computed by PubChem 2.2 (PubChem release 2021.10.14) Dates. Create: 2005-03-26. Modify: 2024-03-30. Description. Propane appears as a colorless gas with a faint petroleum-like odor. It is shipped as a liquefied gas under its vapor pressure. For transportation it may be stenched.
bond energy of c3h8Molecular orbitals of an allyl carbocation. The stability of the allyl carbocation is due to a conjugated π electron system. A “double bond” doesn’t really exist. Instead, it is a group of 3 adjacent, overlapping, non-hybridized p orbitals we call a conjugated π electron system. You can clearly see the interactions between all three of .By checking the geometry of molecules chart above, we have a tetrahedral shape. Now, we move on to the next Carbon. This Carbon has 2 single bonds to 2 Carbons and 2 single bonds to 2 Hydrogens. Again, we have 4 electron groups which result in a tetrahedral. Continuing this trend, we have another tetrahedral with single bonds attached to .

It is a hydrocarbon with two carbon connected with a double bond. In this article, we will study ethene (C2H4) lewis structure, molecular geometry, hybridization, is it polar or non-polar, etc. Ethene gas is lighter than air. It has a sweet odor and can cause an explosion. Also, it is not toxic but a simple asphyxiant. Some properties of Ethene. Write Lewis structures and describe the molecular geometry at each carbon atom in the following compounds: cis-3-hexene; cis-1-chloro-2-bromoethene; 2-pentyne; trans-6 . Write a condensed structural formula, such as CH 3 CH 3, and describe the molecular geometry at each carbon atom. propene; 1-butanol; ethyl propyl ether; .Temperature (K) A B C Reference Comment; 165.81 - 225.98: 3.97488: 795.819-24.884: Powell and Giauque, 1939: Coefficents calculated by NIST from author's data.
propene molecular geometry|bond energy of c3h8
PH0 · which molecule is propene
PH1 · tetrahedral wedge dash
PH2 · propylene electron geometry
PH3 · propene molecular weight
PH4 · molecular geometry of c3h6
PH5 · c3h8 structure line bond
PH6 · c3h8 molecular geometry
PH7 · bond energy of c3h8
PH8 · Iba pa